Way back in elementary school, when you thought of nickel, the first image that popped into your head was probably that nice shiny 5¢ coin. Even though we hardly carry change purses anymore, nickel is still ever-present in our day-to-day lives. From the shiny stainless-steel trim on your building to the knives in your kitchen, nickel is found everywhere. With nickel becoming favoured for use in transport, power generation, cell phones, food preparation, and medical equipment, it leads one to think about where all this nickel is coming from.
The truth is, that high-grade nickel reserves are being readily depleted, and mining companies are looking for new ways to recover this highly desired base metal. I’d like to dig a little deeper into the recovery of nickel, specifically from low-grade nickel ores, and from bleed solutions from the electrorefining of other metals.
The importance of Nickel recovery
Nickel is the fifth most abundant element on Earth. However, the average nickel content of the Earth’s crust is estimated at 0.008 percent. With such low content, it makes it obvious why nickel mining is such an important industry, and why recovery of nickel and nickel recycling are becoming increasingly significant in our consumer-based society.
Nickel has many important properties that contribute to its widespread use in industry; these properties include corrosion resistance, conductive and magnetic properties, and its capability for electromagnetic shielding. Conductive nickel pastes are often used in capacitors: these thin layers of paste are produced by using powders with very small spherical particles, to give tightly packed layers that help increase conductivity. Nickel-coated graphite particles are used for electromagnetic shielding in seals, gaskets, and consumer electronic products such as laptops and phones. The breakdown of primary nickel consumption in the world in 2024 is shown in Figure 1; the end-uses of nickel are shown in Figure 2.
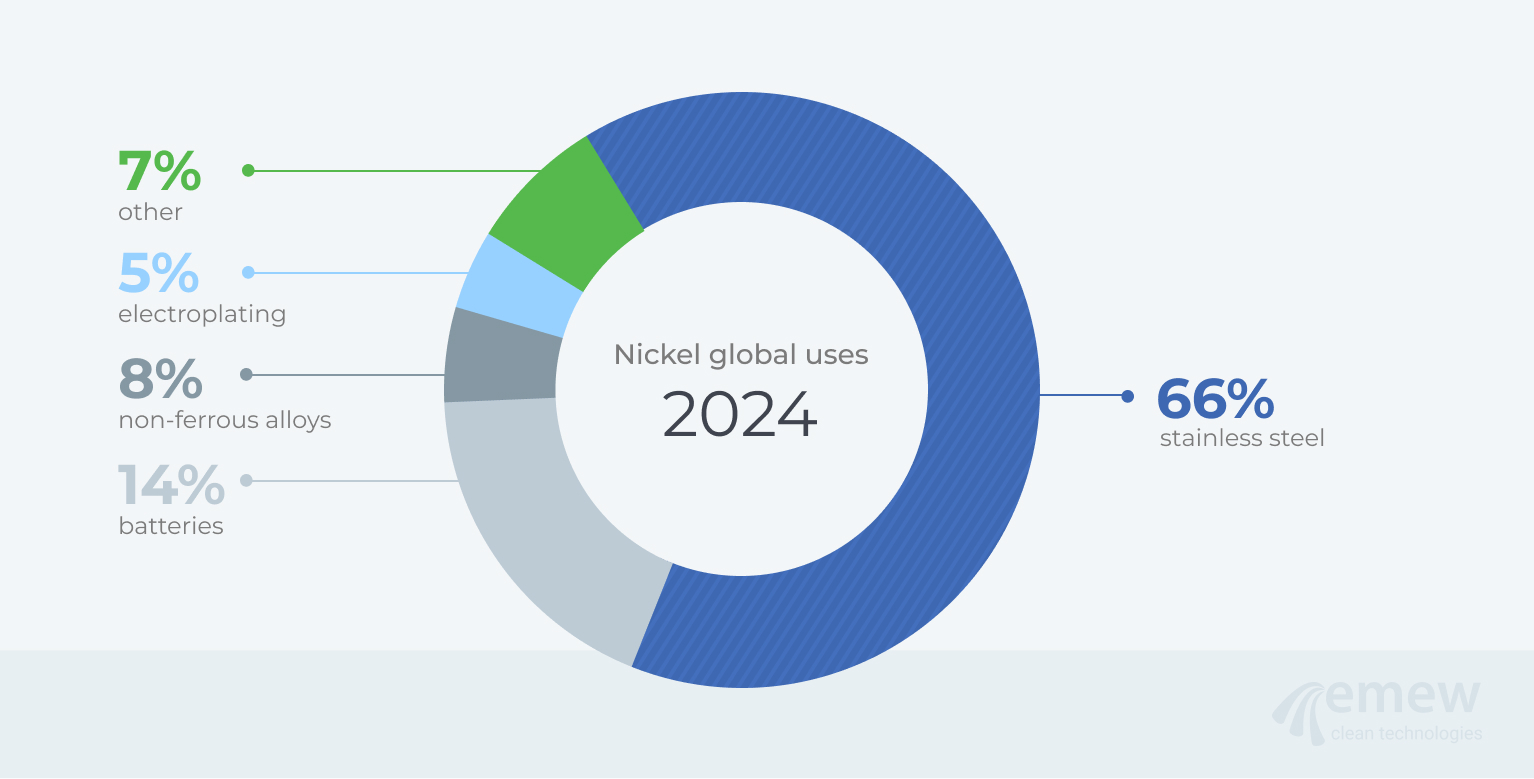
Fig. 1: Primary nickel consumption globally in 20249
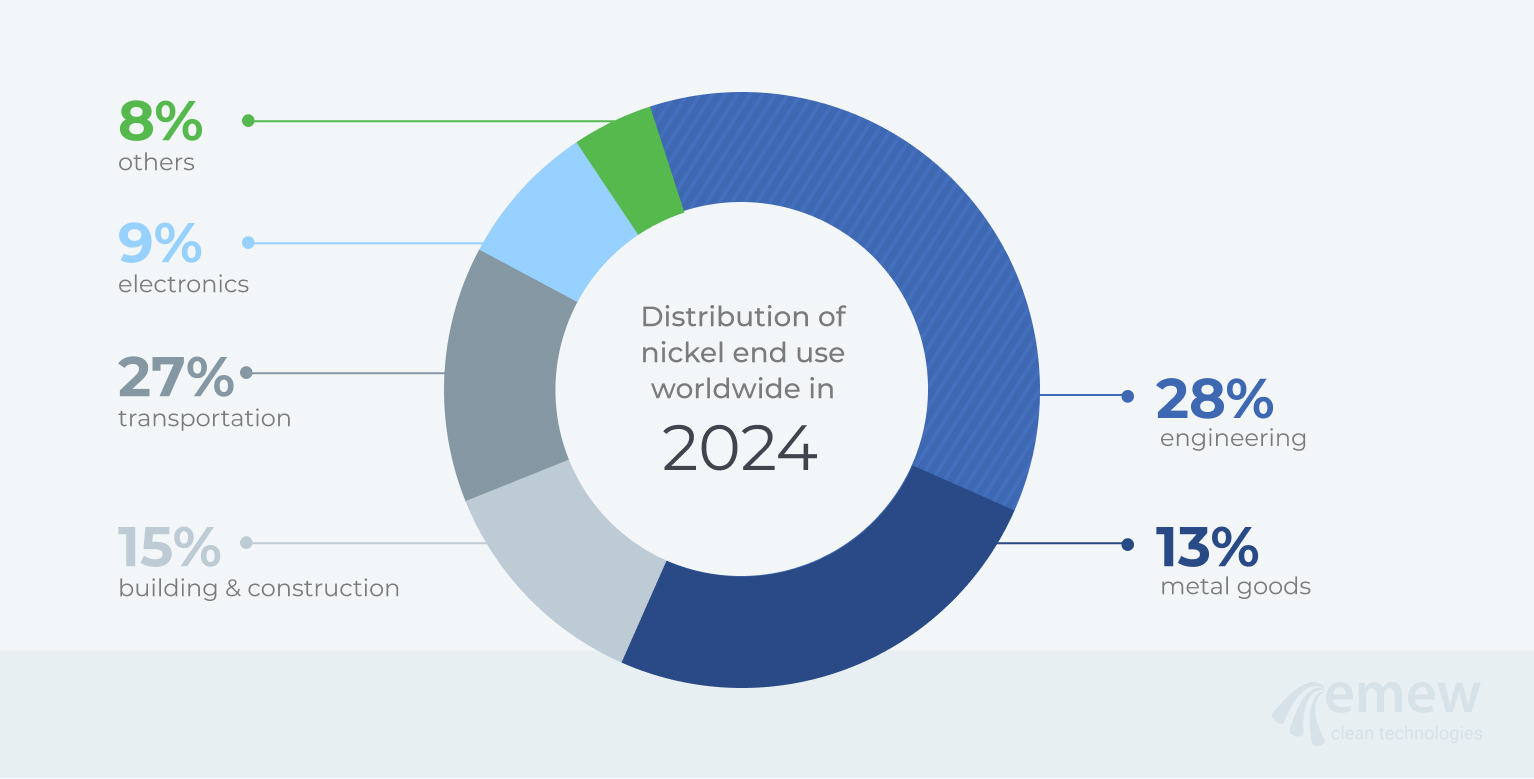
Fig. 2: End-use nickel consumption in 202410
Each year, the global demand for primary nickel exceeds 3.5 million tonnes. Industry experts estimate that 4.4 to 4.6 million tonnes of nickel-bearing scrap are collected and recycled annually. This recycled scrap contains approximately 1 million tonnes of nickel content, meeting about 31% of the total annual global demand.1,2
Production of Nickel from lateritic and sulfide ores
The world’s top nickel producers in 2024 were Indonesia (59.5%), the Philippines (8.9%), Russia (5.7%), Canada (5.1%), and China (3.2%). In 2024, the global production of primary nickel reached 3,526 kt, and it is projected to hit a record 3,735 kt by the end of 2025.1,2
All nickel ores have relatively low nickel content.
Nickel sulfide ores contain nickel as pentlandite, which can be efficiently separated and concentrated using flotation methods. This conventional beneficiation process reduces costs and yields a high-grade nickel concentrate that can be smelted into a saleable product.7
Laterite nickel ores are classified into limonite and saprolite. The limonitic ore is the upper layer, mainly composed of goethite, with nickel content ranging from 0.5% to 1.7%, iron content of 40% to 60%, and low levels of silica (<20%) and magnesia (<10%). The saprolitic ore lies beneath the limonite layer, separated by smectite clay, and has higher nickel grades (1.5% to 3%), lower iron content (<30%), and higher magnesium silicate content. In limonitic ore, nickel replaces iron in the goethite crystal lattice, while in saprolitic ore, nickel and iron replace magnesium in various magnesium silicate minerals, such as garnierite.7
Economic nickel mining grades are being pushed lower by new technologies. Mine grades as 1% nickel are now viable, whereas only a few years ago, grades below 1.5% Nickel were often not viable. For sulfide ores, underground mining and concentrator operations become profitable with combined nickel, copper, and cobalt content above 2%. Surface mining operations tend to be profitable when the combined content of these metals exceeds 1%.5
In 2024, however, cut-off grades as low as 0.5% are being used for laterite ores due to more efficient processing methods becoming available.6
Nickel recovery and techniques of mining Nickel from lateritic deposits
I’d first like to focus on the processing techniques of mining nickel from lateritic deposits, which can be a challenge to execute efficiently. Lateric ores are frequently treated by pyrometallurgical processes using a furnace to smelt the dried ore and carbon as a reducing agent. Sulfur can be added if a matte is required, and further refining can be used to produce ferro-nickel or matte.
Limonitic (oxide) lateric ores are usually treated by hydrometallurgical methods: the Caron Process and Pressure Acid Leaching (PAL), Figure 3. Ores with high levels of magnesium are treated by the Caron process, using selective reduction of the ore, and ammonia leaching. This method is more energy-intensive and produces lower metal recovery
The method of PAL involves preheating the slurried ore and leaching it with concentrated sulfuric acid at high temperatures and pressure. Nickel and cobalt are converted to soluble sulfate salts and are recovered from the slurry in a counter-current decantation circuit (CCD). CCD involves washing the residue and recovering soluble nickel and cobalt. The remaining acid is neutralized using a limestone slurry, which produces a gypsum precipitate. Hydrogen sulfide is injected to precipitate nickel and other sulfides. From here, there is further leaching to remove iron and copper, and finally precipitation of nickel by the addition of ammonia, ammonium sulfate, and hydrogen.
An excellent example of the use of PAL on low-grade lateritic nickel ores is the Ambatovy mine in Madagascar, a joint venture between Sumitomo, and KOMIR This is e f the largest lateritic nickel mines in the world and can produce 60,000 tonnes of refined nickel a year. This mine can also produce 5,600 tonnes of refined cobalt and 210,000 tonnes of ammonium sulfate.

Fig. 3: Flow sheet of the processing of lateritic ores (Natural Environment Research Council, 2008)
Recovery of Nickel and processing of magmatic sulfide ores
The processing of magmatic sulfide ores differs from laterites: sulfide ores are crushed in multiple steps to separate the ore minerals from the gangue. At each stage, the ore is separated by size, using vibrating screens, as well as magnetic separation of iron-rich pyrrhotite. After crushing, the ore is mixed with water to create a slurry and is ground to a powder. Water is then added again, to produce a suspension, and air is blown upwards through the tanks.
Here, chemical separation is used: chemicals are added to make some minerals repel water, allowing the minerals to float to the surface. This froth is then removed, designed to remove copper concentrate. The second stage of froth flotation produces a nickel concentrate of 10-20% Ni, along with other by-products and gangue.
Smelting is the next stage, designed to recover as much metal as possible. Flash smelting is a common method: dry concentrates are fed into a furnace and heated to a liquid matte and slag. From this step, sulfide matte can be recovered, which contains cobalt, and nickel (about 70%). Iron is recovered in the slag, and sulfur as sulfur dioxide. At this stage, pyrometallurgy and hydrometallurgy processes can be used to refine the metals. In pyrometallurgy, metals are separated from the contents of the matte by using heat – to separate based on chemical and physical characteristics such as melting point and density.
In hydrometallurgy, metals are separated based on differences in solubility as well as electrochemical properties. A common method for nickel leaching from the matte is by using ammonia under high pressure, producing a nickel-bearing solution. This solution is heated to remove copper, and the use of hydrogen gas under high pressure precipitates nickel metal; the remaining nickel in the solution can be precipitated by adding hydrogen sulfide. The process steps of the two methods of magmatic sulfide ores can be found in Figure 4. Chlorine and acid leaches can also be used to separate and concentrate the nickel, with the final step of hydrometallurgy being electrowinning.

Fig. 4: Flow sheet of the processing of magmatic sulfide ores (Natural Environment Research Council, 2008)
Enhanced methods of Nickel recovery
New processes are being developed to be more cost-effective and environmentally sound. One example of this is Activox®, which removes the smelting step and uses ultra-fine grinding and pressure oxidation of the concentrate in an autoclave, before solvent extraction and metal precipitation.
Bioleaching is another process that is being looked at for low-grade ores, ideal for reworking waste dumps. This method, under atmospheric pressure, is being used in the Talvivaara project in Finland, a sulfide mineral resource of only 0.27% Ni.
Low-grade ores, like nickel sulfide, are abundant on Earth. Nickel sulfide deposits contain high levels of magnesium silicate gangue minerals (MgO) and can be difficult to process with conventional flotation and smelting technologies. MgO is hydrophilic, which interferes with the flotation of sulfide minerals and reduces flotation recovery. In China, the Jinchuan Group Go. Ltd has 400 Mt of low-grade nickel sulfide mineral ores and uses bioleaching for nickel recovery. This method is interesting because traditional techniques using sulfuric acid are disfavoured since magnesium silicate minerals are so reactive in acidic media - consuming large amounts of acid, which increases operational costs.
High-grade nickel ores are almost exhausted since they are so heavily exploited and less abundant than low-grade ores. High-grade nickel oxides are produced by fluid-bed roasting and chlorine-hydrogen reduction of nickel matte.8 High-purity nickel pellets are produced by using vapour processes; an example of this is the carbonyl (or Mond) process: the copper and precious metals remain as a pyrophoric residue, which requires separate treatment.
Whether high-grade or low-grade, nickel ores need to be processed after mining to upgrade their nickel content from 1-4% Ni to 10-20%. Concentrating the nickel ores usually takes place close to the mine site and involves chemical and physical processes to crush the ore and separate the nickel-bearing and gangue minerals by flotation in the case of sulfidic nickel ores.

Mining nickel directly is the obvious route to obtaining pure nickel; however, nickel can also be recovered as a by-product of other metals. For example, copper electrolysis requires a bleed to eliminate the buildup of impurities that accumulate over time as a result of the electrorefining of impure copper anode to produce a pure metal cathode. A bleed is required when certain impurity concentrations become too high in the electrolyte, thereby adversely affecting the electrorefining performance. The bleed stream is continuously withdrawn and replaced with fresh acid. The amount of bleed and the amount of replacement acid are controlled to keep the sulfuric acid content below 10%. This bleed stream is then further treated, typically by neutralization to precipitate the contained metals.
Nickel can be recovered from the bleed stream of a copper electrorefinery. Classical methods for nickel recovery are precipitation, oxidation, and crystallization. Nickel and sulfate tend to build up in the copper refinery electrolyte and can be removed by crystallization as crude NiSO4·6H2O crystals from the bleed stream after the copper is removed. They are then separated from the mother liquor in a centrifuge, and dried and bagged for shipment.
In most copper refinery electrolytes, the concentration of copper is between 35-60 g/L Cu (typically between 40 and 50 g/L), 120-200 g/L H2SO4 (typically between 150 and 200 g/L), and 0.3-25 g/L Ni. Soluble anode impurities continuously dissolve into the electrolyte, which is why they must be continuously removed from the bleed stream to prevent build-up. Nickel is not the only metal removed from bleed streams; so are arsenic, bismuth, cobalt, iron, antimony, and 1-2% of the copper (since the corrosion of copper plus copper oxides at the anode is faster than the plating at the cathode).
Nickel extraction from bleed streams with SX
Another method for the extraction of nickel from the bleed stream is the use of solvent extraction (SX). Copper-selective solvents can be chosen, and many steps are followed to get nickel and copper powder from the bleed. The following block flow diagram was shown in a paper by A. Agrawal et al. in 2012, demonstrating the steps of SX for nickel and copper recovery, Figure 5.

Fig. 5: Flow diagram for the treatment of copper bleed stream by solvent extraction route (A. Agrawal et al., 2012.)
SX is a tool used in the processing of complex and secondary non-ferrous metal resources due to the ease of metal separation, purification, enrichment, and analysis. Similarly to the process shown above, certain solvents can be used to first extract copper, then adjust the pH to 9-10 for the extraction of nickel. Copper SX plants are common, and they are a closed-loop operation such that they use leaching and electrolysis to produce metal while recycling the reagents in the system. For example, SX can be used to extract and separate nickel and cobalt from chloride, ammoniacal, and sulfate solutions. Queensland Nickel uses an SX route where cobalt is removed as a sulfide by a reagent, then the loaded nickel is stripped with a high concentration of ammonia-ammonium carbonate solution, and the stripped solution is used to produce basic nickel carbonate.
Ammoniacal solutions are used in hydrometallurgical extraction of base metals because iron and manganese are rejected into the residue; solutions without these impurities can undergo metal separation by SX. Some examples of ammoniacal solutions include ocean nodules, nickel laterites, sulfide concentrates, super-alloy scrap and other waste materials, and sulfate solutions.
Sulfuric acid is the most common medium for hydrometallurgical metal extraction; SX processes often report the separation of nickel and cobalt from dilute sulfuric acid solution. This separation is carried out by cation-exchange-type reagents. Ion exchange (IX) is used frequently in metal finishing industries for nickel recovery. Some copper refinery tankhouses also use IX for acid recovery, which involves treatment of the refinery bleed stream from liberator cells with short-bed IX systems to produce a concentrated acid strip for recycling to the tankhouse, and an acid-free byproduct stream. This acid-free stream contains impurity metals, as well as valuable nickel salts, which can be further treated for nickel recovery, using electrowinning to produce nickel metal, or evaporation/crystallization to produce nickel sulfate crystals.
Nickel and Copper powder production through hydrogen reduction
Another method of copper and nickel powder production, reported in 2008 by A. Agrawal et al., uses hydrogen reduction. This method takes the copper bleed and uses hydrogen gas to produce copper powder. The mother liquor with nickel sulfate solution then undergoes precipitation of copper with sodium sulfide to remove the remaining copper, followed by evaporation and crystallization to produce nickel sulfate crystals. The nickel is leached with ammonia, followed by hydrogen reduction to produce nickel powder, Figure 6. Reduction of aqueous metal ions with hydrogen at high temperatures and pressure can produce spherical particles and the hydrogen reduction of nickel can be done from different aqueous solutions.

Fig. 6: Flow diagram for the treatment of copper bleed stream by hydrogen reduction (A. Agrawal et al., 2008.)
Overall, I have only just scratched the surface when it comes to methods of nickel recovery. Since high-grade nickel ores are far and few between these days, fluid-bed roasting and chlorine-hydrogen reduction of nickel matte are becoming less popular techniques. The recovery of nickel from low-grade ores is increasingly more important, from new techniques of pressure oxidation of the concentrate in an autoclave to bioleaching of the nickel in the ore; it is evident that time and money are being put into researching these processes. Solvent extraction and ion exchange are also used in the recovery of nickel from various bleed streams from the electrorefining of other metals, such as copper. The unique properties of nickel, such as corrosion resistance, conductive and magnetic properties, and the capability for electromagnetic shielding, are what make nickel such a valuable resource. In the future, more recovery and recycling of nickel from secondary sources and low-grade ores will be even more important as ore grades decline and primary resources are depleted.
While I have discussed many methods for producing nickel metal, there is an increasing amount of world nickel production that is based on the production of intermediates such as nickel hydroxide, nickel carbonate, and nickel sulfate without producing nickel metal. These nickel intermediates can enter the supply chain more easily for nickel chemical applications such as Li-ion batteries. If the purity is high enough, these intermediates can command a premium over nickel metal price, and many new nickel processes do not make metallic nickel.
Sources
- https://stainless-steel-world.net/the-world-nickel-market-in-2025-a-growing-surplus-in-an-uncertain-global-landscape/
- https://pubs.usgs.gov/periodicals/mcs2025/mcs2025-nickel.pdf
- https://insg.org/index.php/about-nickel/recycling-and-environment/
- https://www.mdpi.com/2673-4605/5/1/104
- https://natural-resources.canada.ca/minerals-mining/mining-data-statistics-analysis/minerals-metals-facts/nickel-facts
- https://www.sciencedirect.com/book/9780080968094/extractive-metallurgy-of-nickel-cobalt-and-platinum-group-metals
- https://ardearesources.com.au/downloads/presentations/arl_p2024050901.pdf
- https://www.scirp.org/journal/paperinformation?paperid=71797
- https://www.sciencedirect.com/book/9781933762166/responsible-care
- https://ar2024.nornickel.com/strategic-report/commodity-markets
- https://www.mordorintelligence.com/industry-reports/nickel-market
Other sources
Agrawal, A., Bagchi, D., Kumari, S., Kumar, V. and Pandey, B. (2008). Hydrogen reduction of bleed stream of an Indian copper industry to produce nickel powder. Materials Letters, 62(17-18), pp.2880-2882.
Agrawal, A., Kumari, S., Parveen, M. and Sahu, K. (2012). Exploitation of Copper Bleed Stream for the Extraction and Recovery of Copper and Nickel by Bis(2,4,4-trimethylpentyl) phosphinic Acid. Mineral Processing and Extractive Metallurgy Review, 33(5), pp.339-351.
Agrawal, A., Manoj, M., Kumari, S., Bagchi, D., Kumar, V. and Pandey, B. (2008). Extractive separation of copper and nickel from copper bleed stream by solvent extraction route. Minerals Engineering, 21(15), pp.1126-1130.
Anon, (2010). Mineral Commodity Summaries: Nickel. [online] Available at: https://minerals.usgs.gov/minerals/pubs/commodity/nickel/mcs-2010-nicke.pdf [Accessed 31 July 2017].
Ambatovy.com. (2014). Ambatovy | Overview. [online] Available at: http://www.ambatovy.com/docs/?p=373 [Accessed 31 Aug. 2017].
Ashcroft, G. (2017). Nickel Laterites: The World’s Largest Source of Nickel. [online] Geology for Investors | Make sense of mining company investments. Available at: https://www.geologyforinvestors.com/nickel-laterites/ [Accessed 31 Aug. 2017]
Cheremisinoff, N. (2007). Handbook of solid waste management and waste minimization technologies. Norwich, NY: Knovel.
Cornwall, H. (1966). Nickel Deposits of North America. [ebook] Washington: United States Government Printing Office. Available at: https://pubs.usgs.gov/bul/1223/report.pdf [Accessed 31 July 2017].
Crundwell, F, Moats, M, Ramachandran, V, Robinson, T, & Davenport, WG 2011, Extractive Metallurgy of Nickel, Cobalt and Platinum Group Metals, Elsevier Science, Oxford. Available from: ProQuest Ebook Central. [31 July 2017].
Encyclopedia Britannica. (2017). laterite | geology. [online] Available at: https://www.britannica.com/science/laterite [Accessed 31 July 2017].
Ikotun, B., Adams, F. and Ikotun, A. (2016). Application of three xanthates collectors on the recovery of nickel and pentlandite in a low-grade nickel sulfide ore using optimum flotation parameters. Particulate Science and Technology, 35(4), pp.462-471.
Jones, J. (2017). Nickel Powders from the Carbonyl Process. [online] AZoM.com. Available at: https://www.azom.com/article.aspx?ArticleID=499 [Accessed 31 July 2017].
Kumar, V., Sahu, S. and Pandey, B. (2010). Prospects for solvent extraction processes in the Indian context for the recovery of base metals. A review. Hydrometallurgy, 103(1-4), pp.45-53.
Natural Environment Research Council (2008). Nickel Mineral Profile. Keyworth, Nottingham, UK: British Geological Survey, pp.1-9. Available at: http://www.bgs.ac.uk/mineralsUK/statistics/mineralProfiles.html
Nickel Institute. (n.d.). Where & Why Nickel is Used. [online] Available at: https://www.nickelinstitute.org/NickelUseInSociety/AboutNickel/WhereWhyNickelIsUsed.aspx [Accessed 31 Aug. 2017].
Schlesinger, M. and Biswas, A. (2011). Extractive metallurgy of copper. Kidlington, Oxford, U.K.: Elsevier.
Sen, P. (2015). T.T. Chen Honorary Symposium on Hydrometallurgy, Electrometallurgy, and Material Characterization, edited by Shije Wang, John E. Dutrizac, Michael L. Free, James Y. Hwang, and Daniel Kim. Materials and Manufacturing Processes, 30(8), pp.1051-1052.
Wang, E. (2016). China imports of Philippine laterite ore hit in year of DENR audit. [online] FastMarkets. Available at: https://www.fastmarkets.com/base-metals-news/asia/2016-review-china-imports-philippine-laterite-ore-hit-year-denr-audit-125904/ [Accessed 31 July 2017].






